Microorganisms dictate our lives, whether we’d like them to or not. Awareness of these tiny microorganisms is often in a negative context. Current events are evidence of this negative association to one particular microorganism, SARS-CoV-2 (the virus that causes COVID-19). We can’t get through a single day without this virus in our minds! However, some microorganisms can have a positive influence on our health in unexpected ways. Early in life, microorganisms (called the ‘microbiome’) found in our gut are beneficial to the brain. But how?
The microbiome is a variety of microorganisms that reside throughout our body. The gut microbiome is made up of a slurry of microorganisms living in the digestive tract (further explained in another Immunobites article). The evidence is continuously building that a microbiome is important for health. Early-life exposure to beneficial microorganisms help shape the immune system and minimize gastrointestinal conditions (for example Crohn’s Disease). Recent research provides additional evidence that the gut microbiome also affects areas beyond the gut: bacteria in the gut can change the activity of brain cells (neurons).
Gut to Brain
Using rodent models, Luck et al. introduced microbiota slurries (consortium) into the guts of neonatal (newly born) mice. In utero, mammals are enclosed in a germ-free (sterile) environment, free from microorganisms. During and shortly after birth, microorganisms are introduced to the newborn mammals. Mice in the study started their lives in germ-free conditions and were given the consortium shortly after birth, while another group remained in the germ-free condition. To test the effects of specific bacteria, the researchers used bacteria from the genus Bifidobacterium, microorganisms that are abundant in human infant gut microbiomes. What, specifically, makes this slurry of Bifidobacterium so important early in life?
Neonatal life coincides with important stages of neurodevelopment. During this time, the brain goes through stages where neurons continuously form and disrupt connections with other neurons (a process called ‘synaptic plasticity’). Over time, these connections (called ‘synapses’) are either strengthened or weakened. Left unchecked, however, synapses can be detrimental if too many exist. It’s important that the brain is able to ‘prune’ these extra connections; a function that’s performed by the immune cells in the brain called microglia. If the brain is under-pruned, the neurons have poor communication abilities.
To capture this stage of neurodevelopment, the researchers studied mice 4 days to 20 days old. An important finding was that germ-free mice had dysfunctional microglia and an increased density of synaptic markers (genes) during this timeframe, suggesting that neurons of these mice had too many connections. Additionally, and as a result, these neurons were worse at communicating. Relative to the germ-free mice, those given the Bifidobacterium microbiome had relatively fewer, but more efficient (functional) connections; signals of a normal nervous system!
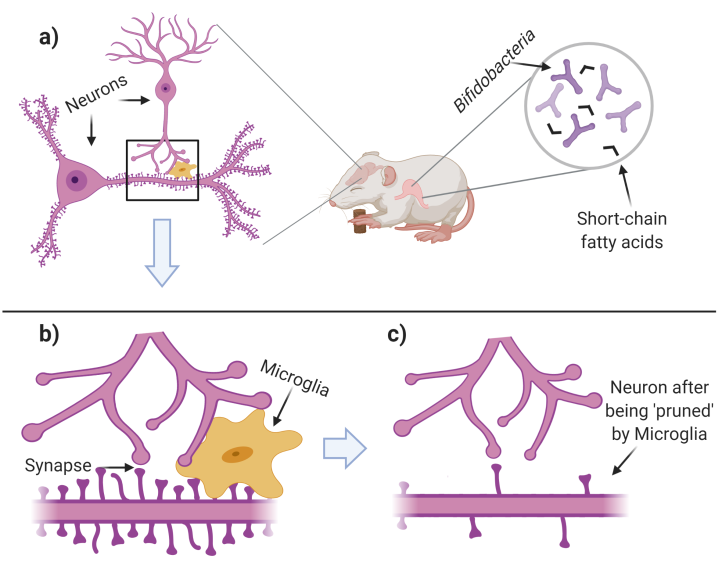
Figure 1. Excessive neuronal connections are ‘pruned’ during normal development. a) Neonatal mice are given Bifidobacteria which produce ‘short-chain fatty acids’. During this time in development, neurons form ‘spines’ (protrusions on the lower neuron) where synapses may form. b) Neurons (purple cells) connect and communicate via synapses. Microglia (gold cell) ‘prune’ the excess synapses and ‘spines’. c) Few, but functional, synapses remain after microglia have finished pruning. Figure created with BioRender.com.
As always, results beget more questions:
This paper suggests that the microbiome consortia is beneficial for neurodevelopment, but answering how the Bifidobacterium microbiome can exert its reach to the brain is an open question. The path from the gut to the brain is a long, obstructed, indirect path. How can bacteria, or any microorganism, have such drastic significance in the brain? Some microorganisms, including the Bifidobacterium species in the study, create small molecules called ‘short-chain fatty acids’ which regulate the development and turnover (‘homeostasis’) of microglia cells. In germ-free conditions, short-chain fatty acids are in short supply, and as a result, microglia don’t follow the normal developmental trajectory.
These collective results shed light on the importance of microorganisms early in life. Bacteria that are populated in the digestive tract shortly after birth aid in normal brain functioning. The released metabolites may travel to the brain and directly act on microglia cells. Microglia cells then remove the excessive number of synapses in the brain found early in life. Research reported here helps us continuously learn about the microorganisms and their far-reaching effects.
Citations:
Rodgers, M. The Mighty, Mighty Microbiome. Retrieved from https://immunobites.com/2018/08/07/the-mighty-mighty-microbiome/
Tamburini, S., Shen, N., Wu, H. et al. The microbiome in early life: implications for health outcomes. Nat Med 22, 713–722 (2016). https://doi.org/10.1038/nm.4142
Luck, B., Engevik, M.A., Ganesh, B.P. et al. Bifidobacteria shape host neural circuits during postnatal development by promoting synapse formation and microglial function. Sci Rep 10, 7737 (2020). https://doi.org/10.1038/s41598-020-64173-3
Erny, D., Hrabě de Angelis, A., Jaitin, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18, 965–977 (2015). https://doi.org/10.1038/nn.4030
Hoyles, L., Snelling, T., Umlai, U. et al. Microbiome–host systems interactions: protective effects of propionate upon the blood–brain barrier. Microbiome 6, 55 (2018). https://doi.org/10.1186/s40168-018-0439-y
The featured image is a cropped image provided by Creative Commons: “Striped grass mouse (zebra mouse)” by Marie Hale is licensed under CC BY 2.0.